
SL Paper 2
Define the term isotopes.
A sample of silicon contains three isotopes.

Calculate the relative atomic mass of silicon using this data.
Describe the structure and bonding in silicon dioxide and carbon dioxide.
Draw the Lewis structure of NH3, state its shape and deduce and explain the H–N–H bond angle in \({\text{N}}{{\text{H}}_{\text{3}}}\).
The graph below shows the boiling points of the hydrides of group 5. Discuss the variation in the boiling points.

Explain, using diagrams, why CO and \({\text{N}}{{\text{O}}_{\text{2}}}\) are polar molecules but \({\text{C}}{{\text{O}}_{\text{2}}}\) is a non-polar molecule.
Markscheme
atoms of the same element with the same atomic number/Z/same number of protons, but different mass numbers/A/different number of neutrons;
\((0.9223 \times 28) + (0.0468 \times 29) + (0.0309 \times 30)\);
28.1/28.11;
Working must be shown to get [2], do not accept 28.09 on its own (given in the data booklet).
Silicon dioxide
single covalent (bonds);
network/giant covalent/ macromolecular / repeating tetrahedral units;
Carbon dioxide
double covalent (bonds);
(simple / discrete) molecular;
Marks may be obtained from suitable structural representations of SiO2 and CO2.
 ;
;
Allow crosses or dots for lone-pair.
trigonal/triangular pyramidal;
(\( \sim \))107° / less than 109.5°;
Do not allow ECF.
LP-BP repulsion \( > \) BP-BP repulsion / one lone pair and three bond pairs / lone pairs/non-bonding pairs repel more than bonding-pairs;
Do not accept repulsion between atoms.
boiling points increase going down the group (from \({\text{P}}{{\text{H}}_{\text{3}}}\) to \({\text{As}}{{\text{H}}_{\text{3}}}\) to \({\text{Sb}}{{\text{H}}_{\text{3}}}\));
\({M_{\text{r}}}\)/number of electrons/molecular size increases down the group;
Accept electron cloud increases down the group for the second marking point.
greater dispersion/London/van der Waal’s forces;
\({\text{N}}{{\text{H}}_{\text{3}}}\)/ammonia has a higher boiling point than expected due to the hydrogen bonding between the molecules;
Do not accept hydrogen bonding alone.
CO:

Award [1] for showing the net dipole moment, or explaining it in words (unsymmetrical distribution of charge).
\(N{O_2}\):

Award [1] for correct representation of the bent shape and [1] for showing the net dipole moment, or explaining it in words (unsymmetrical distribution of charge).
\(C{O_2}\):

Award [1] for correct representation of the linear shape and [1] for showing the two equal but opposite dipoles or explaining it in words (symmetrical distribution of charge).
For all three molecules, allow either arrow or arrow with bar for representation of dipole moment.
Allow correct partial charges instead of the representation of the vector dipole moment.
Ignore incorrect bonds.
Lone pairs not needed.
Examiners report
In general the definition of isotopes was correct in (a) (i), but there are still some candidates who stated “isotopes are elements” and not “atoms of the same element”.
Nearly everybody gave the correct answer of 28.1 for the relative atomic mass of silicon in (ii).
Part (a) (iii) proved to be very difficult for the candidates. There was a lot of confusion about the two molecules; some candidates stated that they had the same double bond. Not many candidates mentioned the giant covalent structure for the silicon dioxide or the simple molecular structure for the carbon dioxide.
In (b) (i) the majority of candidates drew the Lewis structure of the ammonia molecule correctly showing the lone pair of electrons and the correct shape and angle and (ii) was well answered by most candidates.
They realised that \({\text{N}}{{\text{H}}_{\text{3}}}\) had a higher boiling point than \({\text{P}}{{\text{H}}_{\text{3}}}\) because of the intermolecular hydrogen bonding present in \({\text{N}}{{\text{H}}_{\text{3}}}\).
For (c) most answers given here showed diagrams of the three molecules, including distribution of charges, bonding and shapes. Some candidates gave very good answers showing a good understanding of the polarity of molecules.
Boron is most often encountered as a component in borosilicate glass (heat resistant glass).
The naturally occurring element contains two stable isotopes, \(_{\;{\text{5}}}^{{\text{10}}}{\text{B}}\) and \(_{\;{\text{5}}}^{{\text{11}}}{\text{B}}\).
State the number of protons, neutrons and electrons in an atom of \(_{\;{\text{5}}}^{{\text{11}}}{\text{B}}\).

The relative atomic mass of boron is 10.8, to three significant figures. Calculate the percentage of \(_{\;{\text{5}}}^{{\text{10}}}{\text{B}}\) in the naturally occurring element.
Isotopes of boron containing 7 and 8 neutrons also exist. Suggest why releasing isotopes containing more neutrons than the stable isotope into the environment can be dangerous.
(i) State the formula of the compound that boron forms with fluorine.
(ii) Explain why this compound acts as a Lewis acid.
Markscheme
 ;
;
\(10x + 11(100 - x) = 10.8 \times 100\);
\((x = )20\% \);
Award [2] for correct final answer.
Do not allow ECF.
radioactive/radioisotope(s)/give out radiation;
Accept answers that outline the effects of radioactive pollution of the environment.
Do not accept “unstable”.
(i) \({\text{B}}{{\text{F}}_3}\);
(ii) incomplete valence shell / electron deficient / OWTTE;
capable of accepting an electron pair;
Examiners report
This question in general was well answered. Most candidates were able to identify the elementary particles of atomic boron with an encouraging number of students calculating the proportions of the two isotopes. A significant number did leave the question blank however although it should be a familiar example. Most candidates were able to state the formula of boron trifluoride and describe the action of Lewis acids although only a minority could explain its behaviour in terms of boron’s incomplete octet.
This question in general was well answered. Most candidates were able to identify the elementary particles of atomic boron with an encouraging number of students calculating the proportions of the two isotopes. A significant number did leave the question blank however although it should be a familiar example. Most candidates were able to state the formula of boron trifluoride and describe the action of Lewis acids although only a minority could explain its behaviour in terms of boron’s incomplete octet.
This question in general was well answered. Most candidates were able to identify the elementary particles of atomic boron with an encouraging number of students calculating the proportions of the two isotopes. A significant number did leave the question blank however although it should be a familiar example. Most candidates were able to state the formula of boron trifluoride and describe the action of Lewis acids although only a minority could explain its behaviour in terms of boron’s incomplete octet.
This question in general was well answered. Most candidates were able to identify the elementary particles of atomic boron with an encouraging number of students calculating the proportions of the two isotopes. A significant number did leave the question blank however although it should be a familiar example. Most candidates were able to state the formula of boron trifluoride and describe the action of Lewis acids although only a minority could explain its behaviour in terms of boron’s incomplete octet.
Iron rusts in the presence of oxygen and water. Rusting is a redox process involving several steps that produces hydrated iron(III) oxide, \({\text{F}}{{\text{e}}_{\text{2}}}{{\text{O}}_{\text{3}}} \bullet {\text{n}}{{\text{H}}_{\text{2}}}{\text{O}}\), as the final product.
The half-equations involved for the first step of rusting are given below.
Half-equation 1: \({\text{Fe(s)}} \to {\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\)
Half-equation 2: \({{\text{O}}_{\text{2}}}{\text{(aq)}} + {\text{4}}{{\text{e}}^ - } + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{4O}}{{\text{H}}^ - }{\text{(aq)}}\)
A voltaic cell is made from a half-cell containing a magnesium electrode in a solution of magnesium nitrate and a half-cell containing a silver electrode in a solution of silver(I) nitrate.

Hydrogen peroxide decomposes according to the equation below.
\[{\text{2}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}} \to {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} + {{\text{O}}_{\text{2}}}{\text{(g)}}\]
The rate of the decomposition can be monitored by measuring the volume of oxygen gas released. The graph shows the results obtained when a solution of hydrogen peroxide decomposed in the presence of a CuO catalyst.

(i) Identify whether half-equation 1 represents oxidation or reduction, giving a reason for your answer.
(ii) Identify the oxidation number of each atom in the three species in half-equation 2.

(iii) Deduce the overall redox equation for the first step of rusting by combining half-equations 1 and 2.
(iv) Identify the reducing agent in the redox equation in part (iii).
The oxygen in half-equation 2 is atmospheric oxygen that is found dissolved in water in very small concentrations. Explain, in terms of intermolecular forces, why oxygen is not very soluble in water.
(i) Given that magnesium is more reactive than silver, deduce the half-equations for the reactions occurring at each electrode, including state symbols.
Negative electrode (anode):
Positive electrode (cathode):
(ii) Outline one function of the salt bridge.
(i) State the property that determines the order in which elements are arranged in the periodic table.
(ii) State the relationship between the electron arrangement of an element and its group and period in the periodic table.
(i) The experiment is repeated with the same amount of a more effective catalyst, \({\text{Mn}}{{\text{O}}_{\text{2}}}\), under the same conditions and using the same concentration and volume of hydrogen peroxide. On the graph above, sketch the curve you would expect.
(ii) Outline how the initial rate of reaction can be found from the graph.
(iii) Outline a different experimental procedure that can be used to monitor the decomposition rate of hydrogen peroxide.
(iv) A Maxwell–Boltzmann energy distribution curve is drawn below. Label both axes and explain, by annotating the graph, how catalysts increase the rate of reaction.

Markscheme
(i) oxidation and (iron/Fe) loses electrons/increases in oxidation number/state;
(ii)  ;
;
Award [2] for five correct.
Award [1] for four correct.
Accept use of oxidation states (0, +1, –2, –2, +1) for oxidation numbers.
Penalize once for incorrect notation (eg, 2, 2–).
(iii) \({{\text{O}}_2}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}} + {\text{2Fe(s)}} \to {\text{2F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{4O}}{{\text{H}}^ - }{\text{(aq)}}\);
Ignore state symbols.
(iv) Fe/iron;
oxygen is non-polar;
needs to break strong hydrogen bonds/H–bonds between water molecules (to dissolve) / oxygen cannot form hydrogen bonds/H–bonds with water;
oxygen can only form (weak) van der Waals’/vdW/LDF/London/dispersion forces with water;
(i) Negative electrode (anode):
\({\text{Mg(s)}} \to {\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }/\frac{1}{2}{\text{Mg(s)}} \to \frac{1}{2}{\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}} + {{\text{e}}^ - }/\)
\({\text{Mg(s)}} - {\text{2}}{{\text{e}}^ - } \to {\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}}/\frac{1}{2}{\text{Mg(s)}} - {{\text{e}}^ - } \to \frac{1}{2}{\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}}\);
Accept equations for the oxidation of water/hydroxide ions.
Positive electrode (cathode):
\({\text{A}}{{\text{g}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \to {\text{Ag (s)}}\);
Accept Ag equation doubled so that both electrodes involve 2 electrons.
Accept e instead of e–.
Award [1 max] if both equations are correct but the state symbols are missing/incorrect.
Award [1 max] if both equations are reversed but state symbols correct.
(ii) provides ions that flow into electrolytes/half-cells / maintains electrical neutrality of solutions/electrolytes / provides electrical continuity by providing path for migrating ions;
Accept completes the (electrical) circuit / allows current to flow / OWTTE.
(i) atomic number / number of protons;
Accept number of electrons in a (neutral) atom.
(ii) groups indicate the number of electrons in the highest energy level/outer/valence shell;
periods indicate the number of (occupied) energy levels/shells (in the atom);
(i) steeper curve with a similar shape that reaches same maximum volume of \({{\text{O}}_{\text{2}}}\);
(ii) (draw a) tangent to the curve at origin/time = 0/start of reaction;
(calculate) the gradient/slope (of the tangent);
(iii) measure/monitor mass/pressure/\({\text{[}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{]}}\);
Accept measure/monitor temperature of system.
(iv) y-axis: probability / fraction of molecules/particles / probability density
Allow “number of particles/molecules” on y-axis.
and
x-axis: (kinetic) energy;
Accept “speed/velocity” on x-axis.

correct relative position of \({E_{\text{a}}}\) catalysed and \({E_{\text{a}}}\) uncatalysed;
more/greater proportion of molecules/collisions have the lower/required/catalysed \({E_{\text{a}}}\) (and can react upon collision);
M3 can be scored by shading and annotating the graph.
Accept a greater number/proportion of successful collisions as catalyst reduces Ea.
Examiners report
In Part (a) almost all candidates could correctly identify the equation as an oxidation reaction and justify their choice. Assigning oxidation numbers to particular species proved slightly trickier, with many not knowing that elements always have an oxidation state of zero.
Combining the half equations also provided a bit of challenge with many equations having residual electrons, though most students could correctly identify the reducing agent. The aqueous solubility of oxygen gas in Part (b) was poorly explained, with the discussion being most frequently in terms of polarity rather than invoking hydrogen bonding. The electrolysis question in Part (c) was generally well answered, though most relied on “completing the circuit” to obtain the salt bridge mark with few showing any comprehension of the way in which this was achieved. Both the property responsible for the ordering of the periodic table and the relationship of electronic structure to position in the periodic table, required for Part (d), were well known and it was rare for a student not to gain full marks. Similarly in Part (e), most students correctly drew the curve that would result from a more effective catalyst. Many also seemed to be aware of the basic idea of how to find the reaction rate, though correct use of the terms “tangent” and “gradient” was rare and many failed to note it referred to “initial rate”. Most students could also identify an appropriate alternative method for monitoring the rate. In the final section most students could accurately label the axes of a Maxwell-Boltzmann curve and many could also use it to explain the effect of a catalyst, though some weaker students confused this with the effect of temperature and constructed a second curve.
In Part (a) almost all candidates could correctly identify the equation as an oxidation reaction and justify their choice. Assigning oxidation numbers to particular species proved slightly trickier, with many not knowing that elements always have an oxidation state of zero.
Combining the half equations also provided a bit of challenge with many equations having residual electrons, though most students could correctly identify the reducing agent. The aqueous solubility of oxygen gas in Part (b) was poorly explained, with the discussion being most frequently in terms of polarity rather than invoking hydrogen bonding. The electrolysis question in Part (c) was generally well answered, though most relied on “completing the circuit” to obtain the salt bridge mark with few showing any comprehension of the way in which this was achieved. Both the property responsible for the ordering of the periodic table and the relationship of electronic structure to position in the periodic table, required for Part (d), were well known and it was rare for a student not to gain full marks. Similarly in Part (e), most students correctly drew the curve that would result from a more effective catalyst. Many also seemed to be aware of the basic idea of how to find the reaction rate, though correct use of the terms “tangent” and “gradient” was rare and many failed to note it referred to “initial rate”. Most students could also identify an appropriate alternative method for monitoring the rate. In the final section most students could accurately label the axes of a Maxwell-Boltzmann curve and many could also use it to explain the effect of a catalyst, though some weaker students confused this with the effect of temperature and constructed a second curve.
In Part (a) almost all candidates could correctly identify the equation as an oxidation reaction and justify their choice. Assigning oxidation numbers to particular species proved slightly trickier, with many not knowing that elements always have an oxidation state of zero.
Combining the half equations also provided a bit of challenge with many equations having residual electrons, though most students could correctly identify the reducing agent. The aqueous solubility of oxygen gas in Part (b) was poorly explained, with the discussion being most frequently in terms of polarity rather than invoking hydrogen bonding. The electrolysis question in Part (c) was generally well answered, though most relied on “completing the circuit” to obtain the salt bridge mark with few showing any comprehension of the way in which this was achieved. Both the property responsible for the ordering of the periodic table and the relationship of electronic structure to position in the periodic table, required for Part (d), were well known and it was rare for a student not to gain full marks. Similarly in Part (e), most students correctly drew the curve that would result from a more effective catalyst. Many also seemed to be aware of the basic idea of how to find the reaction rate, though correct use of the terms “tangent” and “gradient” was rare and many failed to note it referred to “initial rate”. Most students could also identify an appropriate alternative method for monitoring the rate. In the final section most students could accurately label the axes of a Maxwell-Boltzmann curve and many could also use it to explain the effect of a catalyst, though some weaker students confused this with the effect of temperature and constructed a second curve.
In Part (a) almost all candidates could correctly identify the equation as an oxidation reaction and justify their choice. Assigning oxidation numbers to particular species proved slightly trickier, with many not knowing that elements always have an oxidation state of zero.
Combining the half equations also provided a bit of challenge with many equations having residual electrons, though most students could correctly identify the reducing agent. The aqueous solubility of oxygen gas in Part (b) was poorly explained, with the discussion being most frequently in terms of polarity rather than invoking hydrogen bonding. The electrolysis question in Part (c) was generally well answered, though most relied on “completing the circuit” to obtain the salt bridge mark with few showing any comprehension of the way in which this was achieved. Both the property responsible for the ordering of the periodic table and the relationship of electronic structure to position in the periodic table, required for Part (d), were well known and it was rare for a student not to gain full marks. Similarly in Part (e), most students correctly drew the curve that would result from a more effective catalyst. Many also seemed to be aware of the basic idea of how to find the reaction rate, though correct use of the terms “tangent” and “gradient” was rare and many failed to note it referred to “initial rate”. Most students could also identify an appropriate alternative method for monitoring the rate. In the final section most students could accurately label the axes of a Maxwell-Boltzmann curve and many could also use it to explain the effect of a catalyst, though some weaker students confused this with the effect of temperature and constructed a second curve.
In Part (a) almost all candidates could correctly identify the equation as an oxidation reaction and justify their choice. Assigning oxidation numbers to particular species proved slightly trickier, with many not knowing that elements always have an oxidation state of zero.
Combining the half equations also provided a bit of challenge with many equations having residual electrons, though most students could correctly identify the reducing agent. The aqueous solubility of oxygen gas in Part (b) was poorly explained, with the discussion being most frequently in terms of polarity rather than invoking hydrogen bonding. The electrolysis question in Part (c) was generally well answered, though most relied on “completing the circuit” to obtain the salt bridge mark with few showing any comprehension of the way in which this was achieved. Both the property responsible for the ordering of the periodic table and the relationship of electronic structure to position in the periodic table, required for Part (d), were well known and it was rare for a student not to gain full marks. Similarly in Part (e), most students correctly drew the curve that would result from a more effective catalyst. Many also seemed to be aware of the basic idea of how to find the reaction rate, though correct use of the terms “tangent” and “gradient” was rare and many failed to note it referred to “initial rate”. Most students could also identify an appropriate alternative method for monitoring the rate. In the final section most students could accurately label the axes of a Maxwell-Boltzmann curve and many could also use it to explain the effect of a catalyst, though some weaker students confused this with the effect of temperature and constructed a second curve.
A sample of magnesium contains three isotopes: magnesium-24, magnesium-25 and magnesium-26, with abundances of 77.44%, 10.00% and 12.56% respectively.
Phosphorus(V) oxide, \({{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}{\text{ }}({M_{\text{r}}} = 283.88)\), reacts vigorously with water \(({M_{\text{r}}} = 18.02)\), according to the equation below.
\[{{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}{\text{(s)}} + {\text{6}}{{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{4}}{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\]
Calculate the relative atomic mass of this sample of magnesium correct to two decimal places.
Predict the relative atomic radii of the three magnesium isotopes, giving your reasons.
Describe the bonding in magnesium.
State an equation for the reaction of magnesium oxide with water.
A student added 5.00 g of \({{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}\) to 1.50 g of water. Determine the limiting reactant, showing your working.
Calculate the mass of phosphoric(V) acid, \({{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}\), formed in the reaction.
State a balanced equation for the reaction of aqueous \({{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}\) with excess aqueous sodium hydroxide, including state symbols.
State the formula of the conjugate base of \({{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}\).
(i) Deduce the Lewis structure of \({\text{PH}}_4^ + \).
(ii) Predict, giving a reason, the bond angle around the phosphorus atom in \({\text{PH}}_4^ + \).
(iii) Predict whether or not the P–H bond is polar, giving a reason for your choice.
Markscheme
\(\left( {\frac{{(77.44 \times 24) + (10.00 \times 25) + (12.56{\text{ }}26)}}{{100}}} \right)\);
24.35;
Award [2] for correct final answer.
Two decimal places are required for M2.
Do not award any marks for 24.31 without showing method (as the value can be copied from the Data Booklet).
same atomic radii / 160 pm;
isotopes only differ by number of neutrons/size of nucleus / radius
determined by electron shells and number of protons / OWTTE;
Accept neutrons do not affect distance of electrons / OWTTE.
(lattice of) positive ions/cations and mobile/free/delocalized electrons;
Accept “sea of electrons” instead of “delocalized electrons”.
Award M1 for a suitable diagram.
electrostatic attraction (between ions and delocalized electrons);
\({\text{MgO}} + {{\text{H}}_{\text{2}}}{\text{O}} \to {\text{Mg(OH}}{{\text{)}}_{\text{2}}}/{\text{M}}{{\text{g}}^{2 + }} + {\text{2O}}{{\text{H}}^ - }\);
Accept reversible arrow.
\({{\text{P}}_4}{{\text{O}}_{10}}{\text{: }}\left( {\frac{{{\text{5.00}}}}{{{\text{283.88}}}} = } \right){\text{ 0.0176 (mol)}}\) and \({{\text{H}}_2}{\text{O: }}\left( {\frac{{{\text{1.50}}}}{{{\text{18.02}}}} = } \right){\text{ 0.0832 (mol)}}\);
\({{\text{H}}_2}{\text{O}}\) is the limiting reactant and reason related to stoichiometry;
\(\frac{{0.0832 \times 4}}{6}/0.0555{\text{ (mol)}}\);
\((0.0555 \times 98.00 = ){\text{ }}5.44{\text{ g}}\);
The unit is needed for M2.
Award [2] for correct final answer.
Do not penalize slight numerical variations due to premature rounding.
\({{\text{H}}_3}{\text{P}}{{\text{O}}_4}{\text{(aq)}} + {\text{3NaOH(aq)}} \to {\text{N}}{{\text{a}}_3}{\text{P}}{{\text{O}}_4}{\text{(aq)}} + {\text{3}}{{\text{H}}_2}{\text{O(l)}}\)
correct products and balancing;
correct state symbols;
Accept valid ionic equations.
\({{\text{H}}_2}{\text{PO}}_4^ - \);
(i)  ;
;
Accept dots, crosses or lines for pairs of electrons.
No need to distinguish the dative covalent bond from the other bonds.
Charge is required for the mark.
Do not penalize missing square brackets.
(ii) \(109^\circ 27'/109.5^\circ /109^\circ \);
4 electron domains/pairs/(negative) charge centres (around central atom/P);
Accept ion is tetrahedral / electron pairs/domains repel each other.
(iii) non-polar and P and H have the same electronegativity / OWTTE;
Accept slightly polar as precise electronegativities of P and H are not identical / OWTTE.
Examiners report
In Part (a) most candidates gained full marks, with the most common error being a failure to quote the answer to the precision specified, but the explanations of deflection, and more particularly detection, in the mass spectrometer were weak. The prediction of relative atomic radii of the isotopes, something that required the application of reason rather than recall, also proved much more challenging. Part (b) revealed that many candidates have a very weak understanding of the metallic bond with many thinking the bonding was ionic.
Even when they knew about a cation lattice and delocalized electrons, a mark was frequently dropped by failing to specify that the attraction between them was electrostatic. Most candidates wrote the correct equation in Part (c), but it is still disturbing that some students at this level cannot write even the most straightforward chemical equation. In Part (d) many students proved capable of carrying out routine stoichiometric calculations to identify the limiting reactant and use the result to find the mass of the product.
Even if the final result was incorrect quite frequently students gained some credit through the application of ECF. Only the better candidates could write an equation for the neutralisation of phosphoric(V) acid and even the routine derivation of a conjugate base from the formula of the acid proved difficult for many. In Part (e) most students could manage the correct Lewis structure, though some lost the mark through omitting the charge. Many candidates also scored well on the shape of the ion and the polarity of the P-H bond.
In Part (a) most candidates gained full marks, with the most common error being a failure to quote the answer to the precision specified, but the explanations of deflection, and more particularly detection, in the mass spectrometer were weak. The prediction of relative atomic radii of the isotopes, something that required the application of reason rather than recall, also proved much more challenging. Part (b) revealed that many candidates have a very weak understanding of the metallic bond with many thinking the bonding was ionic.
Even when they knew about a cation lattice and delocalized electrons, a mark was frequently dropped by failing to specify that the attraction between them was electrostatic. Most candidates wrote the correct equation in Part (c), but it is still disturbing that some students at this level cannot write even the most straightforward chemical equation. In Part (d) many students proved capable of carrying out routine stoichiometric calculations to identify the limiting reactant and use the result to find the mass of the product.
Even if the final result was incorrect quite frequently students gained some credit through the application of ECF. Only the better candidates could write an equation for the neutralisation of phosphoric(V) acid and even the routine derivation of a conjugate base from the formula of the acid proved difficult for many. In Part (e) most students could manage the correct Lewis structure, though some lost the mark through omitting the charge. Many candidates also scored well on the shape of the ion and the polarity of the P-H bond.
In Part (a) most candidates gained full marks, with the most common error being a failure to quote the answer to the precision specified, but the explanations of deflection, and more particularly detection, in the mass spectrometer were weak. The prediction of relative atomic radii of the isotopes, something that required the application of reason rather than recall, also proved much more challenging. Part (b) revealed that many candidates have a very weak understanding of the metallic bond with many thinking the bonding was ionic.
Even when they knew about a cation lattice and delocalized electrons, a mark was frequently dropped by failing to specify that the attraction between them was electrostatic. Most candidates wrote the correct equation in Part (c), but it is still disturbing that some students at this level cannot write even the most straightforward chemical equation. In Part (d) many students proved capable of carrying out routine stoichiometric calculations to identify the limiting reactant and use the result to find the mass of the product.
Even if the final result was incorrect quite frequently students gained some credit through the application of ECF. Only the better candidates could write an equation for the neutralisation of phosphoric(V) acid and even the routine derivation of a conjugate base from the formula of the acid proved difficult for many. In Part (e) most students could manage the correct Lewis structure, though some lost the mark through omitting the charge. Many candidates also scored well on the shape of the ion and the polarity of the P-H bond.
In Part (a) most candidates gained full marks, with the most common error being a failure to quote the answer to the precision specified, but the explanations of deflection, and more particularly detection, in the mass spectrometer were weak. The prediction of relative atomic radii of the isotopes, something that required the application of reason rather than recall, also proved much more challenging. Part (b) revealed that many candidates have a very weak understanding of the metallic bond with many thinking the bonding was ionic.
Even when they knew about a cation lattice and delocalized electrons, a mark was frequently dropped by failing to specify that the attraction between them was electrostatic. Most candidates wrote the correct equation in Part (c), but it is still disturbing that some students at this level cannot write even the most straightforward chemical equation. In Part (d) many students proved capable of carrying out routine stoichiometric calculations to identify the limiting reactant and use the result to find the mass of the product.
Even if the final result was incorrect quite frequently students gained some credit through the application of ECF. Only the better candidates could write an equation for the neutralisation of phosphoric(V) acid and even the routine derivation of a conjugate base from the formula of the acid proved difficult for many. In Part (e) most students could manage the correct Lewis structure, though some lost the mark through omitting the charge. Many candidates also scored well on the shape of the ion and the polarity of the P-H bond.
In Part (a) most candidates gained full marks, with the most common error being a failure to quote the answer to the precision specified, but the explanations of deflection, and more particularly detection, in the mass spectrometer were weak. The prediction of relative atomic radii of the isotopes, something that required the application of reason rather than recall, also proved much more challenging. Part (b) revealed that many candidates have a very weak understanding of the metallic bond with many thinking the bonding was ionic.
Even when they knew about a cation lattice and delocalized electrons, a mark was frequently dropped by failing to specify that the attraction between them was electrostatic. Most candidates wrote the correct equation in Part (c), but it is still disturbing that some students at this level cannot write even the most straightforward chemical equation. In Part (d) many students proved capable of carrying out routine stoichiometric calculations to identify the limiting reactant and use the result to find the mass of the product.
Even if the final result was incorrect quite frequently students gained some credit through the application of ECF. Only the better candidates could write an equation for the neutralisation of phosphoric(V) acid and even the routine derivation of a conjugate base from the formula of the acid proved difficult for many. In Part (e) most students could manage the correct Lewis structure, though some lost the mark through omitting the charge. Many candidates also scored well on the shape of the ion and the polarity of the P-H bond.
In Part (a) most candidates gained full marks, with the most common error being a failure to quote the answer to the precision specified, but the explanations of deflection, and more particularly detection, in the mass spectrometer were weak. The prediction of relative atomic radii of the isotopes, something that required the application of reason rather than recall, also proved much more challenging. Part (b) revealed that many candidates have a very weak understanding of the metallic bond with many thinking the bonding was ionic.
Even when they knew about a cation lattice and delocalized electrons, a mark was frequently dropped by failing to specify that the attraction between them was electrostatic. Most candidates wrote the correct equation in Part (c), but it is still disturbing that some students at this level cannot write even the most straightforward chemical equation. In Part (d) many students proved capable of carrying out routine stoichiometric calculations to identify the limiting reactant and use the result to find the mass of the product.
Even if the final result was incorrect quite frequently students gained some credit through the application of ECF. Only the better candidates could write an equation for the neutralisation of phosphoric(V) acid and even the routine derivation of a conjugate base from the formula of the acid proved difficult for many. In Part (e) most students could manage the correct Lewis structure, though some lost the mark through omitting the charge. Many candidates also scored well on the shape of the ion and the polarity of the P-H bond.
In Part (a) most candidates gained full marks, with the most common error being a failure to quote the answer to the precision specified, but the explanations of deflection, and more particularly detection, in the mass spectrometer were weak. The prediction of relative atomic radii of the isotopes, something that required the application of reason rather than recall, also proved much more challenging. Part (b) revealed that many candidates have a very weak understanding of the metallic bond with many thinking the bonding was ionic.
Even when they knew about a cation lattice and delocalized electrons, a mark was frequently dropped by failing to specify that the attraction between them was electrostatic. Most candidates wrote the correct equation in Part (c), but it is still disturbing that some students at this level cannot write even the most straightforward chemical equation. In Part (d) many students proved capable of carrying out routine stoichiometric calculations to identify the limiting reactant and use the result to find the mass of the product.
Even if the final result was incorrect quite frequently students gained some credit through the application of ECF. Only the better candidates could write an equation for the neutralisation of phosphoric(V) acid and even the routine derivation of a conjugate base from the formula of the acid proved difficult for many. In Part (e) most students could manage the correct Lewis structure, though some lost the mark through omitting the charge. Many candidates also scored well on the shape of the ion and the polarity of the P-H bond.
In Part (a) most candidates gained full marks, with the most common error being a failure to quote the answer to the precision specified, but the explanations of deflection, and more particularly detection, in the mass spectrometer were weak. The prediction of relative atomic radii of the isotopes, something that required the application of reason rather than recall, also proved much more challenging. Part (b) revealed that many candidates have a very weak understanding of the metallic bond with many thinking the bonding was ionic.
Even when they knew about a cation lattice and delocalized electrons, a mark was frequently dropped by failing to specify that the attraction between them was electrostatic. Most candidates wrote the correct equation in Part (c), but it is still disturbing that some students at this level cannot write even the most straightforward chemical equation. In Part (d) many students proved capable of carrying out routine stoichiometric calculations to identify the limiting reactant and use the result to find the mass of the product.
Even if the final result was incorrect quite frequently students gained some credit through the application of ECF. Only the better candidates could write an equation for the neutralisation of phosphoric(V) acid and even the routine derivation of a conjugate base from the formula of the acid proved difficult for many. In Part (e) most students could manage the correct Lewis structure, though some lost the mark through omitting the charge. Many candidates also scored well on the shape of the ion and the polarity of the P-H bond.
In Part (a) most candidates gained full marks, with the most common error being a failure to quote the answer to the precision specified, but the explanations of deflection, and more particularly detection, in the mass spectrometer were weak. The prediction of relative atomic radii of the isotopes, something that required the application of reason rather than recall, also proved much more challenging. Part (b) revealed that many candidates have a very weak understanding of the metallic bond with many thinking the bonding was ionic.
Even when they knew about a cation lattice and delocalized electrons, a mark was frequently dropped by failing to specify that the attraction between them was electrostatic. Most candidates wrote the correct equation in Part (c), but it is still disturbing that some students at this level cannot write even the most straightforward chemical equation. In Part (d) many students proved capable of carrying out routine stoichiometric calculations to identify the limiting reactant and use the result to find the mass of the product.
Even if the final result was incorrect quite frequently students gained some credit through the application of ECF. Only the better candidates could write an equation for the neutralisation of phosphoric(V) acid and even the routine derivation of a conjugate base from the formula of the acid proved difficult for many. In Part (e) most students could manage the correct Lewis structure, though some lost the mark through omitting the charge. Many candidates also scored well on the shape of the ion and the polarity of the P-H bond.
The element boron has two naturally occurring isotopes, \(^{{\text{10}}}{\text{B}}\) and \(^{{\text{11}}}{\text{B}}\).
Define the term isotopes of an element.
Calculate the percentage abundance of each isotope, given that the relative atomic mass of B is 10.81.
Deduce the Lewis structures of \({\text{N}}{{\text{H}}_{\text{3}}}\) and \({\text{B}}{{\text{F}}_{\text{3}}}\).
\[{\text{N}}{{\text{H}}_{\text{3}}}\quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad {\text{B}}{{\text{F}}_{\text{3}}}\]
Describe how covalent bonds are formed.
Compare the shapes of the two molecules and explain the difference using valence shell electron pair repulsion theory (VSEPR).
Predict and explain whether the molecules \({\text{N}}{{\text{H}}_{\text{3}}}\) and \({\text{B}}{{\text{F}}_{\text{3}}}\) are polar molecules.
Markscheme
atoms of the same element/with the same number of protons/with same atomic
number but different number of neutrons/mass number/mass;
\(10x + 11(1 - x) = 10.81,{\text{ }}x = 0.19\);
Accept similar method.
10B: 19% and 11B: 81%;

Accept any combination of lines, dots or crosses to represent electron pairs.
sharing of electrons between atoms;
\({\text{N}}{{\text{H}}_{\text{3}}}\): (trigonal/triangular) pyramidal;
\({\text{B}}{{\text{F}}_{\text{3}}}\): trigonal/triangular planar;
\({\text{N}}{{\text{H}}_{\text{3}}}\) has 4 negative centres of charge/three bonding pairs and one lone pair and \({\text{B}}{{\text{F}}_{\text{3}}}\) has 3 negative centres of charge/three bonding pairs / OWTTE;
(bond angles) 107° in \({\text{N}}{{\text{H}}_{\text{3}}}\) and 120° in \({\text{B}}{{\text{F}}_{\text{3}}}\);
Accept 107.5° for NH3.
\({\text{B}}{{\text{F}}_{\text{3}}}\) not polar as no net dipole moment / BF bond polarities cancel each other out / symmetrical distribution of charge;
\({\text{N}}{{\text{H}}_{\text{3}}}\) polar as net dipole moment present / NH bond polarities do not cancel each other out / unsymmetrical distribution of charge;
Accept suitable diagram showing dipole moments.
Do not accept electronegativities cancel out.
Examiners report
Few candidates defined isotopes in terms of atoms.
The percentage abundance was generally done well.
The Lewis structure of \({\text{N}}{{\text{H}}_{\text{3}}}\) was well answered, though many forgot the non-bonding electron pairs of fluorine.
The covalent bond was often just described as electron sharing between non-metals.
Shapes of molecules and angles were often well known, but the explanation using the VSEPR theory was very weak, with many students not being able to describe the bonding and lone pairs in terms of negative charge centres.
Polarity was very poorly understood, with almost no candidates actually talking about polarity of bonds or showing an understanding of the impact of symmetry on the overall dipole moment.
Lithium and boron are elements in period 2 of the periodic table. Lithium occurs in group 1 (the alkali metals) and boron occurs in group 3. Isotopes exist for both elements.
Every element has its own unique line emission spectrum.
(i) Define the terms atomic number, mass number and isotopes of an element.
Atomic number:
Mass number:
Isotopes of an element:
(ii) Distinguish between the terms group and period.
(iii) Deduce the electron arrangements of the lithium ion, \({\text{L}}{{\text{i}}^ + }\), and the boron atom, B.
\({\text{L}}{{\text{i}}^ + }\):
B:
(iv) Naturally occurring boron exists as two isotopes with mass numbers of 10 and 11. Calculate the percentage abundance of the lighter isotope, using this information and the relative atomic mass of boron in Table 5 of the Data Booklet.
v) Lithium exists as two isotopes with mass numbers of 6 and 7. Deduce the number of protons, electrons and neutrons for each isotope.

(i) Distinguish between a continuous spectrum and a line spectrum.
(ii) Draw a diagram to show the electron transitions between energy levels in a hydrogen atom that are responsible for the two series of lines in the ultraviolet and visible regions of the spectrum. Label your diagram to show three transitions for each series.
(i) Explain why metals are good conductors of electricity and why they are malleable.
(ii) Iron is described as a transition metal. Identify the two most common ions of iron.
iii) Deduce the chemical formulas of lithium oxide and iron(II) oxide.
Lithium oxide:
Iron(II) oxide:
Markscheme
(i) Atomic number:
number of protons (in nucleus/atom);
Mass number:
(sum of) number of protons and neutrons (in nucleus/atom);
Isotopes of an element:
atoms of same element / atoms with same number of protons/atomic number/Z but different number of neutrons/mass number/A;
Penalize once only use of the term element in the three definitions, for example, number of protons in an element or number of protons and neutrons in an element or element with the same atomic number but different mass number.
(ii) Group: (elements in vertical) columns in periodic table and Period: (elements in horizontal) rows in periodic table;
Allow elements in same group have similar chemical properties and within a period, atoms have same number of shells/energy levels (but number of electrons in valence/outer shell increases).
Allow groups distributed vertically and periods distributed horizontally / OWTTE.
Allow group number gives number of valence/outer shell electrons (for maingroup elements) and period gives same number of shells/energy levels.
(iii) Li+: 2/1s2;
B: 2,3/1s22s22p1;
(iv) correct mathematical expression set-up \({\text{(e.g. }}\left( {\frac{x}{{100}}} \right)(10) + \left[ {\frac{{(100 - x)}}{{(100)}}} \right](11) = 10.81)\);
19%;
Award [2] for correct final answer.
(v) 
Award [1 max] for correct number of neutrons for both isotopes if numbers of protons or electrons is not given.
Award [1 max] for correct number of protons and electrons for both isotopes if number of neutrons is not given or if numbers of neutrons are incorrect.
(i) Continuous spectrum: radiation spread over all wavelengths/frequencies/energies/colours / OWTTE;
Line spectrum: radiation (absorbed/emitted) at certain/specific wavelengths/frequencies/energies/colours / OWTTE;
Allow series of (separate/discrete) lines which converge/get closer together at high energy / OWTTE.
(ii) 
showing y-axis labelled as energy/E or labelling at least two energy levels
(\(n = 1\), \(n = 2\) etc. but not for \(n = 0\));
showing energy levels converging;
showing jumps to \(n = 1\) for ultraviolet series;
showing jumps to \(n = 2\) for visible series;
UV and visible must be labelled.
(i) metals have delocalized electrons / sea of electrons which are mobile/can move / OWTTE;
layers/positive ions/cations/atoms slide past/over each other / OWTTE;
Do not accept nuclei for M2.
(ii) Fe2+ and Fe3+ ;
(iii) Lithium oxide: Li2O and Iron(II) oxide: FeO;
Examiners report
Many candidates defined the atomic number, mass number and isotopes correctly although the weaker candidates incorrectly used the term element instead of atom and others defined mass number in terms of molar mass instead of sum of protons and neutrons in the nucleus. Distinguishing between a group and a period and deducing the electron arrangements of Li+ and boron was handled well by majority of candidates. Many candidates struggled to calculate the percentage abundance of the lighter isotope whereas in part (v), most candidates correctly deduced the number of protons, neutrons and electrons in the two isotopes of lithium.
Distinguishing between a continuous and line spectrum in part (b) proved difficult for many candidates. Similarly, drawing a diagram to show the electron transitions between energy levels in a hydrogen atom was challenging for many candidates. Common errors seen were: starting incorrectly at \(n = 0\), not showing convergence or mixed up between the ultraviolet and visible lines.
In Part (c), although the explanation of why metals are good conductors of electricity was answered well, some candidates did not refer to delocalized or sea of electrons. Explanation of why metals are malleable proved to be difficult for many candidates. Identifying the two most common ions of iron and deducing chemical formulas was correctly answered by majority of the candidates.
Iron has three main naturally occurring isotopes which can be investigated using a mass spectrometer.
A sample of iron has the following isotopic composition by mass.

Calculate the relative atomic mass of iron based on this data, giving your answer to two decimal places.
Calculate the number of electrons in the ion \(^{{\text{56}}}{\text{F}}{{\text{e}}^{2 + }}\).
Describe the bonding in iron and explain the electrical conductivity and malleability of the metal.
Markscheme
\(\frac{{(54 \times 5.95) + (56 \times 91.88) + (57{\text{ }}2.17)}}{{100}}\);
55.90;
Award [2] for correct final answer.
Answer must be to 2 d.p.
24;
metallic (bonding);
positive ions/cations and delocalized/sea of electrons;
electrostatic attraction between the two;
Award [2 max] for description of bonding
Conductivity:
electrons delocalised/free to move;
Malleability:
atoms/ions/cations can move without breaking bonds / atoms/ions/cations can slide
past each other;
Examiners report
Most candidates could correctly calculate the relative atomic mass although a few lost a mark by giving their answers to 1 or 3 decimal places.
Most candidates correctly calculated the number of electrons, but the most frequent incorrect answers were 28 and 54.
The explanation of iron‟s properties was well answered in terms of metallic bonding and most candidates correctly described its electrical conductivity as due to free flowing electrons. However, only a few could explain malleability in terms of the layers of ions being able to slide over each other.
The relative atomic mass of naturally occurring copper is 63.55. Calculate the abundances of \(^{{\text{63}}}{\text{Cu}}\) and \(^{{\text{65}}}{\text{Cu}}\) in naturally occurring copper.
The isotopes of some elements are radioactive. State a radioisotope used in medicine.
State a balanced equation for the reaction of sodium with water. Include state symbols.
With reference to electronic arrangements, suggest why the reaction between rubidium and water is more vigorous than that between sodium and water.
Describe and explain what you will see if chlorine gas is bubbled through a solution of
(i) potassium iodide.
(ii) potassium fluoride.
Markscheme
\(63x + 65(1 - x) = 63.55\);
(or some other mathematical expression).
\(^{63}{\text{Cu}} = 72.5\% \) and \(^{65}{\text{Cu}} = 27.5\% \);
Allow 63Cu = 0.725 and 65Cu = 0.275.
Award [2] for correct final answer.
60Co /131I /125I;
Must contain correct mass numbers.
Allow other formats such as cobalt-60, Co-60 etc.
Award no marks if a correct radioisotope is given with an incorrect radioisotope.
Allow any other radioisotope if you can verify its use.
\({\text{2Na(s)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}} \to {\text{2NaOH(aq)}} + {{\text{H}}_2}{\text{(g) / Na(s)}} + {{\text{H}}_2}{\text{O(l)}} \to {\text{NaOH(aq)}} + \frac{1}{2}{{\text{H}}_2}{\text{(g)}}\)
Award [1] for correct balanced equation.
Award [1] for correct state symbols for sodium, water, sodium hydroxide and hydrogen.
Second mark is not dependent on equation being correctly balanced.
(Rb more reactive because) electron lost further from nucleus so less tightly held;
Rb electron is in 5th energy level and (Na less reactive) as electron lost in 3rd energy level / OWTTE;
Allow [1 max] for electron arrangements of Na (e.g. 2,8,1) and Rb if second mark is not scored.
(i) solution becomes yellow/orange/brown/darker;
chlorine is more reactive than iodine (and displaces it from solution) / OWTTE;
Allow correct equation (KI + Cl2 \( \to \) KCl + I2) for second mark or stating that iodine/I2 is formed.
(ii) no colour change/nothing happens as fluorine is more reactive than chlorine / OWTTE;
Examiners report
Candidates who knew how to calculate the abundances of Cu-63 and Cu-65 generally scored full marks, but many had no idea at all on how to approach the question in (c).
Surprisingly very few candidates were able to state a radioisotope used in medicine. C-13 and C-14 were often given and sometimes elements were suggested but with no specified mass number.
Approximately 25% of candidates got the equation mark, but many gave incorrect state symbols. A significant number of candidates wrote equations with the formation of Na2O or even atomic H.
Some of the weaker candidates explained the reactivity by referring to the change in reactivity down group 1 with no further explanation. Many referred to the increased number of shells in Rb or the increased distance the valence electron is from the nucleus, but some did not go on to explain that this affected its attraction/ease of loss. Very few candidates scored the marks for reference to valence electrons being in the third and fifth shells respectively.
The colour change in (i) was usually known. There was rarely any explanation in (ii) as to why there is no observable reaction with the fluoride.
Calcium carbide, CaC2, is an ionic solid.
Describe the nature of ionic bonding.
State the electron configuration of the Ca2+ ion.
When calcium compounds are introduced into a gas flame a red colour is seen; sodium compounds give a yellow flame. Outline the source of the colours and why they are different.
Suggest two reasons why solid calcium has a greater density than solid potassium.
Outline why solid calcium is a good conductor of electricity.
Calcium carbide reacts with water to form ethyne and calcium hydroxide.
CaC2(s) + H2O(l) → C2H2(g) + Ca(OH)2(aq)
Estimate the pH of the resultant solution.
Markscheme
electrostatic attraction AND oppositely charged ions
[1 mark]
1s22s22p63s23p6
OR
[Ar]
[1 mark]
«promoted» electrons fall back to lower energy level
energy difference between levels is different
Accept “Na and Ca have different nuclear charge” for M2.
[2 marks]
Any two of:
stronger metallic bonding
smaller ionic/atomic radius
two electrons per atom are delocalized
OR
greater ionic charge
greater atomic mass
Do not accept just “heavier” or “more massive” without reference to atomic mass.
[2 marks]
delocalized/mobile electrons «free to move»
[1 mark]
pH > 7
Accept any specific pH value or range of values above 7 and below 14.
[1 mark]
Examiners report
\(^{{\text{131}}}{\text{I}}\) is a radioactive isotope of iodine.
Define the term isotope.
Determine the number of neutrons in one atom of iodine-131.
Markscheme
atoms which have same atomic number but different mass number / atoms of the same element which have different numbers of neutrons / atoms with the same number of protons but different numbers of neutrons / atom of an element with a fixed number of protons but a number of neutrons which can be variable;
78;
Examiners report
In (a)(i), the word atoms was frequently omitted from the definition; it is accepted that it would have been preferable to ask for the definition of isotopes of an element as specified in the syllabus.
The answer in (ii) was generally correct.
2-methylbutan-2-ol, \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{C(OH)C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\), is a liquid with a smell of camphor that was formerly used as a sedative. One way of producing it starts with 2-methylbut-2-ene.
2-chloro-2-methylbutane contains some molecules with a molar mass of approximately \({\text{106 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) and some with a molar mass of approximately \({\text{108 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Draw the structure of 2-methylbut-2-ene.
State the other substances required to convert 2-methylbut-2-ene to 2-methylbutan-2-ol.
Explain whether you would expect 2-methylbutan-2-ol to react with acidified potassium dichromate(VI).
Explain why 2-methylbut-2-ene is less soluble in water than 2-methylbutan-2-ol.
Outline why there are molecules with different molar masses.
Markscheme
 ;
;
Accept condensed formula such as (CH3)2CCHCH3.
water/\({{\text{H}}_{\text{2}}}{\text{O}}\);
Accept steam.
(concentrated) sulfuric acid/\({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\) (catalyst);
Accept phosphoric acid/H3PO4.
Award [2] for HBr and NaOH, (2 stage process via the halogenoalkane).
not react;
tertiary alcohol (not easily oxidized);
2-methylbutan-2-ol has hydroxyl/OH group;
Do not accept “hydroxide group”.
Allow 2-methylbutan-2-ol is an alcohol.
2-methylbutan-2-ol can form H-bonds (to water) / 2-methylbut-2-ene cannot form H-bonds (to water);
chlorine can be \(^{{\text{35}}}{\text{Cl}}\)/Cl–35 or \(^{{\text{37}}}{\text{Cl}}\)/Cl–37;
Accept “chlorine can exist as two isotopes”.
Answer must refer to chlorine rather than isotopes in general.
Examiners report
This was the second most popular question answered in Section B. This question was focussed on organic chemistry and attempted by many candidates.
Most candidates were able to draw the correct structure of 2-methylbut-2-ene in part (a). In part (b), water and sulfuric acid were stated correctly as the reagents. In part (c), most candidates knew that tertiary alcohols do not react. In part (d), the most common mistake was some candidates thinking that the hydroxyl group in an alcohol was a hydrogen bond. Some other candidates could not write that the alcohol forms hydrogen bonds with water. In part (e), many candidates got \({{\text{S}}_{\text{N}}}{\text{1}}\), though an odd few candidates identified the mechanism as \({{\text{S}}_{\text{N}}}{\text{2}}\). In part (e) (ii), the mechanisms proved a problem for several candidates. The use of curly arrows in reaction mechanisms continues to be poorly understood, the arrow often pointing in the wrong direction. Candidates must take care to accurately draw the position of the curly arrows illustrating the movement of electrons. Some candidates forgot to include the lone pair for the curly arrow going from the lone pair on O to \({{\text{C}}^ + }\). Some candidates had the lone pair incorrectly located on the H and others had the curly arrow going to an atom instead of between the O and the \({{\text{C}}^ + }\). Part (iii) was well answered.
Part (f) proved challenging for candidates and very few referred to chlorines isotopes. In addition, the majority of candidates did not state that the same rate could be applied as the isotopes have the same chemical properties. In part (g), many candidates scored three out of five marks. Some candidates forgot to state that the sample is converted to the gaseous state for the vaporization stage. Many candidates although knew about detection but only few stated that the ions hit the counter and an electrical signal is generated.
This was the second most popular question answered in Section B. This question was focussed on organic chemistry and attempted by many candidates.
Most candidates were able to draw the correct structure of 2-methylbut-2-ene in part (a). In part (b), water and sulfuric acid were stated correctly as the reagents. In part (c), most candidates knew that tertiary alcohols do not react. In part (d), the most common mistake was some candidates thinking that the hydroxyl group in an alcohol was a hydrogen bond. Some other candidates could not write that the alcohol forms hydrogen bonds with water. In part (e), many candidates got \({{\text{S}}_{\text{N}}}{\text{1}}\), though an odd few candidates identified the mechanism as \({{\text{S}}_{\text{N}}}{\text{2}}\). In part (e) (ii), the mechanisms proved a problem for several candidates. The use of curly arrows in reaction mechanisms continues to be poorly understood, the arrow often pointing in the wrong direction. Candidates must take care to accurately draw the position of the curly arrows illustrating the movement of electrons. Some candidates forgot to include the lone pair for the curly arrow going from the lone pair on O to \({{\text{C}}^ + }\). Some candidates had the lone pair incorrectly located on the H and others had the curly arrow going to an atom instead of between the O and the \({{\text{C}}^ + }\). Part (iii) was well answered.
Part (f) proved challenging for candidates and very few referred to chlorines isotopes. In addition, the majority of candidates did not state that the same rate could be applied as the isotopes have the same chemical properties. In part (g), many candidates scored three out of five marks. Some candidates forgot to state that the sample is converted to the gaseous state for the vaporization stage. Many candidates although knew about detection but only few stated that the ions hit the counter and an electrical signal is generated.
This was the second most popular question answered in Section B. This question was focussed on organic chemistry and attempted by many candidates.
Most candidates were able to draw the correct structure of 2-methylbut-2-ene in part (a). In part (b), water and sulfuric acid were stated correctly as the reagents. In part (c), most candidates knew that tertiary alcohols do not react. In part (d), the most common mistake was some candidates thinking that the hydroxyl group in an alcohol was a hydrogen bond. Some other candidates could not write that the alcohol forms hydrogen bonds with water. In part (e), many candidates got \({{\text{S}}_{\text{N}}}{\text{1}}\), though an odd few candidates identified the mechanism as \({{\text{S}}_{\text{N}}}{\text{2}}\). In part (e) (ii), the mechanisms proved a problem for several candidates. The use of curly arrows in reaction mechanisms continues to be poorly understood, the arrow often pointing in the wrong direction. Candidates must take care to accurately draw the position of the curly arrows illustrating the movement of electrons. Some candidates forgot to include the lone pair for the curly arrow going from the lone pair on O to \({{\text{C}}^ + }\). Some candidates had the lone pair incorrectly located on the H and others had the curly arrow going to an atom instead of between the O and the \({{\text{C}}^ + }\). Part (iii) was well answered.
Part (f) proved challenging for candidates and very few referred to chlorines isotopes. In addition, the majority of candidates did not state that the same rate could be applied as the isotopes have the same chemical properties. In part (g), many candidates scored three out of five marks. Some candidates forgot to state that the sample is converted to the gaseous state for the vaporization stage. Many candidates although knew about detection but only few stated that the ions hit the counter and an electrical signal is generated.
This was the second most popular question answered in Section B. This question was focussed on organic chemistry and attempted by many candidates.
Most candidates were able to draw the correct structure of 2-methylbut-2-ene in part (a). In part (b), water and sulfuric acid were stated correctly as the reagents. In part (c), most candidates knew that tertiary alcohols do not react. In part (d), the most common mistake was some candidates thinking that the hydroxyl group in an alcohol was a hydrogen bond. Some other candidates could not write that the alcohol forms hydrogen bonds with water. In part (e), many candidates got \({{\text{S}}_{\text{N}}}{\text{1}}\), though an odd few candidates identified the mechanism as \({{\text{S}}_{\text{N}}}{\text{2}}\). In part (e) (ii), the mechanisms proved a problem for several candidates. The use of curly arrows in reaction mechanisms continues to be poorly understood, the arrow often pointing in the wrong direction. Candidates must take care to accurately draw the position of the curly arrows illustrating the movement of electrons. Some candidates forgot to include the lone pair for the curly arrow going from the lone pair on O to \({{\text{C}}^ + }\). Some candidates had the lone pair incorrectly located on the H and others had the curly arrow going to an atom instead of between the O and the \({{\text{C}}^ + }\). Part (iii) was well answered.
Part (f) proved challenging for candidates and very few referred to chlorines isotopes. In addition, the majority of candidates did not state that the same rate could be applied as the isotopes have the same chemical properties. In part (g), many candidates scored three out of five marks. Some candidates forgot to state that the sample is converted to the gaseous state for the vaporization stage. Many candidates although knew about detection but only few stated that the ions hit the counter and an electrical signal is generated.
This was the second most popular question answered in Section B. This question was focussed on organic chemistry and attempted by many candidates.
Most candidates were able to draw the correct structure of 2-methylbut-2-ene in part (a). In part (b), water and sulfuric acid were stated correctly as the reagents. In part (c), most candidates knew that tertiary alcohols do not react. In part (d), the most common mistake was some candidates thinking that the hydroxyl group in an alcohol was a hydrogen bond. Some other candidates could not write that the alcohol forms hydrogen bonds with water. In part (e), many candidates got \({{\text{S}}_{\text{N}}}{\text{1}}\), though an odd few candidates identified the mechanism as \({{\text{S}}_{\text{N}}}{\text{2}}\). In part (e) (ii), the mechanisms proved a problem for several candidates. The use of curly arrows in reaction mechanisms continues to be poorly understood, the arrow often pointing in the wrong direction. Candidates must take care to accurately draw the position of the curly arrows illustrating the movement of electrons. Some candidates forgot to include the lone pair for the curly arrow going from the lone pair on O to \({{\text{C}}^ + }\). Some candidates had the lone pair incorrectly located on the H and others had the curly arrow going to an atom instead of between the O and the \({{\text{C}}^ + }\). Part (iii) was well answered.
Part (f) proved challenging for candidates and very few referred to chlorines isotopes. In addition, the majority of candidates did not state that the same rate could be applied as the isotopes have the same chemical properties. In part (g), many candidates scored three out of five marks. Some candidates forgot to state that the sample is converted to the gaseous state for the vaporization stage. Many candidates although knew about detection but only few stated that the ions hit the counter and an electrical signal is generated.
Rubidium contains two stable isotopes, \(^{{\text{85}}}{\text{Rb}}\) and \(^{{\text{87}}}{\text{Rb}}\). The relative atomic mass of rubidium is given in Table 5 of the Data Booklet.
Calculate the percentage of each isotope in pure rubidium. State your answers to three significant figures.
State the number of electrons and the number of neutrons present in an atom of \(^{{\text{87}}}{\text{Rb}}\).
Number of electrons:
Number of neutrons:
Markscheme
(let \(x = {\text{fraction of}}{{\text{ }}^{{\text{85}}}}{\text{Rb}}\))
\(\frac{{(x \times 85) + [(100 - x) \times 87]}}{{100}} = 85.47\);
\(^{{\text{85}}}{\text{Rb}} = 76.5\% \) and \(^{{\text{87}}}{\text{Rb}} = 23.5\% \);
Award [2] for correct final answer.
37 (electrons);
50 (neutrons);
Examiners report
This question was answered very well by those that knew the correct mathematical technique; however some candidates did not have any idea how to tackle this problem.
The vast majority of candidates could correctly state the number of electrons and neutrons present in Rubidium- 87.
Isotopes are atoms of the same element with different mass numbers. Two isotopes of cobalt are Co-59 and Co-60.
Deduce the missing information and complete the following table.

Markscheme

Award [2] for all four correct.
Award [1] for two or three correct.
Examiners report
This was generally well answered and sub-atomic particles were well known although some gave 53 instead of 125 and Co was often given instead of I.
Chlorine occurs in Group 7, the halogens.
Two stable isotopes of chlorine are \(^{{\text{35}}}{\text{Cl}}\) and \(^{{\text{37}}}{\text{Cl}}\) with mass numbers 35 and 37 respectively.
Chlorine has an electronegativity value of 3.2 on the Pauling scale.
Chloroethene, H2C=CHCl, the monomer used in the polymerization reaction in the manufacture of the polymer poly(chloroethene), PVC, can be synthesized in the following two-stage reaction pathway.
\[\begin{array}{*{20}{l}} {{\text{Stage 1:}}}&{{{\text{C}}_2}{{\text{H}}_4}{\text{(g)}} + {\text{C}}{{\text{l}}_2}{\text{(g)}} \to {\text{ClC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{Cl(g)}}} \\ {{\text{Stage 2:}}}&{{\text{ClC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{Cl(g)}} + {\text{HC=CHCl(g)}} + {\text{HCl(g)}}} \end{array}\]
Define the term isotopes of an element.
Calculate the number of protons, neutrons and electrons in the isotopes 35Cl and 37Cl.

Using the mass numbers of the two isotopes and the relative atomic mass of chlorine from Table 5 of the Data Booklet, determine the percentage abundance of each isotope.
Percentage abundance 35Cl:
Percentage abundance 37Cl:
Define the term electronegativity.
Using Table 7 of the Data Booklet, explain the trends in electronegativity values of the Group 7 elements from F to I.
State the balanced chemical equation for the reaction of potassium bromide, KBr(aq), with chlorine, Cl2(aq).
Describe the colour change likely to be observed in this reaction.
Determine the enthalpy change, \(\Delta H\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for stage 1 using average bond enthalpy data from Table 10 of the Data Booklet.
State whether the reaction given in stage 1 is exothermic or endothermic.
Draw the structure of poly(chloroethene) showing two repeating units.
Suggest why monomers are often gases or volatile liquids whereas polymers are solids.
Markscheme
atoms of same element / atoms with same number of protons/atomic number/Z;
Do not allow elements instead of atoms in second alternative.
(but) different numbers of neutrons/mass number/A;

Allow [1 max] for 17 p, 17 e for both if n’s are omitted or incorrect.
Allow [1 max] for 35Cl: 18 n and 37Cl: 20 n if p’s and e’s are omitted.
\(({\text{for}}{{\text{ }}^{{\text{35}}}}{\text{Cl}}:x\% ){\text{ }}35x + 3700 - 37x = 3545\);
Allow other alternative mathematical arrangements.
\(^{{\text{35}}}{\text{Cl}} = 77.5\% \) and \(^{{\text{37}}}{\text{Cl}} = 22.5\% \);
Award [1 max] for correct percentages if no correct working is shown.
ability of atom/nucleus to attract bonding/shared pair of electrons / attraction of nucleus for bonding/shared pair of electrons / OWTTE;
Do not allow element instead of atom/nucleus.
increasing atomic radii (down the group) / OWTTE;
so reduced attraction (for the bonding electrons) / OWTTE;
screening/shielding effect of inner electrons / OWTTE;
Allow more energy levels/electron shells for M1.
Do not accept decrease in nuclear charge.
\({\text{2KBr(aq)}} + {\text{C}}{{\text{l}}_2}{\text{(aq)}} \to {\text{2KCl(aq)}} + {\text{B}}{{\text{r}}_2}{\text{(aq)}}\);
Ignore state symbols.
Allow ionic equation.
colourless/pale yellow/green to yellow/orange/brown;
Start and end colours must both be mentioned.
Bonds breaking:
1 \( \times \) (C=C) \( + \) 4 \( \times \) (C–H) \( + \) 1 \( \times \) (Cl–Cl)
\( = (1)(612) + (4)(413) + (1)(243)/ = ( + )2507{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Bonds forming:
1 \( \times \) (C–C) \( + \) 4 \( \times \) (C–H) \( + \) 2 \( \times \) (Cl–Cl)
\( = (1)(347) + (4)(413) + (2)(346)/ = - 2691{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Enthalpy change:
\((2507 - 2691 = ){\text{ }} - 184{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
OR
Bonds breaking:
1 \( \times \) (C=C) \( + \) 1 \( \times \) (Cl–Cl)
\( = (1)(612) + (1)(243)/ = ( + )855{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Bonds forming:
1 \( \times \) (C–C) \( + \) 2 \( \times \) (C–Cl)
\( = (1)(347) + (2)(346)/ = - 1039{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Enthalpy change:
\((855 - 1039 = ){\text{ }} - 184{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
exothermic;
Do not award mark unless based on some value for part (iii).
representation of PVC showing two repeating units;
For example,

Brackets not necessary but continuation bonds must be given.
No penalty if chlorines are not on same side.
No penalty if chlorines are on two middle C atoms or on two end C atoms.
monomers are smaller molecules / monomers have smaller mass / smaller surface area than polymers;
weaker/fewer intermolecular/London/dispersion/van der Waals’ forces (of attraction);
Allow reverse argument.
Allow abbreviation for London/dispersion as FDL or for van der Waals’ as vdW.
Award zero if reference is made to breaking of bonds.
Examiners report
This was by far the most popular choice of question in Section B. Again, part a) (i) proved challenging as many candidates failed to refer to atoms in their definition and scored only 1 mark out of 2.
In a) (ii) most candidates could state the numbers of protons, neutrons and electrons in the isotopes of chlorine. Those who got this wrong gave answers which indicated a complete lack of understanding of atomic structure.
In a) (iii) some candidates remembered the percentage abundance of chlorine isotopes but could not do the calculation.
Part b) (i) required another definition. Again, many candidates lost marks for inarticulate responses.
The explanation in b) (ii) of trends in electronegativity values was reasonably well done, with most candidates scoring at least one mark out of two.
However, writing a balanced equation in b) (iii) was poorly done with many candidates not knowing the formula of KCl, and not knowing what products would be formed. This is clearly on the syllabus in 3.3.1.
Almost no-one knew the colours of aqueous chlorine and aqueous bromine in b) (iv).
In part c) (ii) the calculation of \(\Delta H\) using bond enthalpies was done well. Some candidates failed to use the C=C bond enthalpy value and some did not recall that bond breaking is endothermic and bond formation exothermic.
Nearly everyone scored a mark in c) (iii) as follow-through marks were awarded.
Drawing two repeating units of poly(chloroethene) presented difficulties in c) (iv). Some candidates tried to draw the monomers joined through the chlorine atoms.
In c) (v) most candidates scored at least one out of two for explaining why monomers have a much lower melting point than polymers.
Carbon and silicon belong to the same group of the periodic table.
Both silicon and carbon form oxides.
State the period numbers of both carbon and silicon.
Describe and compare three features of the structure and bonding in the three allotropes of carbon: diamond, graphite and \({{\text{C}}_{{\text{60}}}}\) fullerene.
Draw the Lewis structure of \({\text{C}}{{\text{O}}_{\text{2}}}\) and predict its shape and bond angle.
Describe the structure and bonding in \({\text{Si}}{{\text{O}}_{\text{2}}}\).
Explain why silicon dioxide is a solid and carbon dioxide is a gas at room temperature.
Describe the bonding within the carbon monoxide molecule.
Silicon has three stable isotopes, \(^{{\text{28}}}{\text{Si}}\), \(^{{\text{29}}}{\text{Si}}\) and \(^{{\text{30}}}{\text{Si}}\). The heaviest isotope, \(^{{\text{30}}}{\text{Si}}\), has a percentage abundance of 3.1%. Calculate the percentage abundance of the lightest isotope to one decimal place.
Markscheme
C: 2 and Si: 3;
Award [2 max] for three of the following features:
Bonding
Graphite and C60 fullerene: covalent bonds and van der Waals’/London/dispersion forces;
Diamond: covalent bonds (and van der Waals’/London/dispersion forces);
Delocalized electrons
Graphite and C60 fullerene: delocalized electrons;
Diamond: no delocalized electrons;
Structure
Diamond: network/giant structure / macromolecular / three-dimensional structure and Graphite: layered structure / two-dimensional structure / planar;
C60 fullerene: consists of molecules / spheres made of atoms arranged in hexagons/pentagons;
Bond angles
Graphite: 120° and Diamond: 109°;
C60 fullerene: bond angles between 109–120°;
Allow Graphite: sp2 and Diamond: sp3.
Allow C60 fullerene: sp2 and sp3.
Number of atoms each carbon is bonded to
Graphite and C60 fullerene: each C atom attached to 3 others;
Diamond: each C atom attached to 4 atoms / tetrahedral arrangement of C (atoms);

linear and 180°;
Accept crosses, lines or dots as electron pairs.
network/giant structure / macromolecular;
each Si atom bonded covalently to 4 oxygen atoms and each O atom bonded covalently to 2 Si atoms / single covalent bonds;
Award [1 max] for answers such as network-covalent, giant-covalent or macromolecular-covalent.
Both M1 and M2 can be scored by a suitable diagram.
Silicon dioxide: strong/covalent bonds in network/giant structure/macromolecule;
Carbon dioxide: weak/van der Waals’/dispersion/London forces between molecules;
triple (covalent) bond;
one electron pair donated by oxygen to carbon atom / dative (covalent)/coordinate (covalent) bond;
Award [1 max] for representation of C\(\equiv\)O.
Award [2] if CO shown with dative covalent bond.
\(2809 = 3.10 \times 30 + 28x + 29(96.9 - x)\);
\(\% {{\text{ }}^{28}}{\text{Si}} = (93 + 2810.1 - 2809) = 94.1\% \);
Award [2] for correct final answer.
Examiners report
Part (b) was exceptionally well done.
Many candidates struggled with Part (c) not being able to clearly identify the bonding and structure in the allotropes of carbon. Candidates often incorrectly discussed the properties of the allotropes.
In Part (d), candidates were competent at drawing carbon dioxide but struggled to identify the bonding and structure in silicon dioxide.
Most candidates incorrectly identifying silicon dioxide as molecular compound.
Candidates also struggled to explain why \({\text{C}}{{\text{O}}_{\text{2}}}\) was a gas and \({\text{Si}}{{\text{O}}_{\text{2}}}\) was a solid a room temperature and again commented on the properties of the compounds rather than the structure and bonding.
In part (e) many candidates failed to state that a dative bond was present in CO.
Although the calculation in (f) was more challenging than similar questions in the past, it was managed by many candidates.
Draw and label an energy level diagram for the hydrogen atom. In your diagram show how the series of lines in the ultraviolet and visible regions of its emission spectrum are produced, clearly labelling each series.
Markscheme

showing y-axis labelled as energy/E / labelling at least two energy levels;
showing a minimum of four energy levels/lines with convergence;
showing jumps to n = 1 for ultraviolet series;
showing jumps to n = 2 for visible light series;
Must show at least two vertical lines per series to score M3 and M4 but penalize once only.
For M3, M4 if transition not shown from higher to lower energy level penalize only once.
Examiners report
Although this generally proved to be the second most difficult question in Section A there were some excellent diagrams with some even linking a correct energy level diagram with a correct line emission spectrum. Candidates in some schools, however, appeared not to have encountered these ideas at all. Common errors were to label the first energy level as n = 0 rather than n = 1 and to only include one transition for each series. Sometimes the arrows showed the absorption rather than the required emission transition.
A sample of vaporized elemental magnesium is introduced into a mass spectrometer.
One of the ions that reaches the detector is \(^{{\text{26}}}{\text{M}}{{\text{g}}^ + }\).
Calculate the number of protons, neutrons and electrons in the \(^{{\text{26}}}{\text{M}}{{\text{g}}^ + }\) ion.
The sample contained the three isotopes \(^{{\text{24}}}{\text{Mg}}\), \(^{{\text{25}}}{\text{Mg}}\) and \(^{{\text{26}}}{\text{Mg}}\). The relative percentage abundances of \(^{{\text{25}}}{\text{Mg}}\) and \(^{{\text{26}}}{\text{Mg}}\) are 10.00% and 11.01% respectively. Calculate the relative atomic mass \({\text{(}}{{\text{A}}_r}{\text{)}}\) of magnesium, accurate to two decimal places.
Markscheme
Protons: 12
Neutrons: 14
Electrons: 11
Award [2] for three correct answers.
Award [1] for two correct answers.
Award [0] for one correct answer.
\(^{{\text{24}}}{\text{Mg}} = (100 - 10.00 - 11.01 = ){\text{ }}78.99\% \);
\({A_r} = (24 \times 0.7899 + 25 \times 0.1000 + 26 \times 0.1101 = ){\text{ }}24.32\);
Award [2] for correct final answer which must be to two decimal places.
Do not accept data booklet value of 24.31.
Examiners report
The question on the structure of atoms generally scored well. The workings of the mass spectrometer was less well known; with many getting confused with the role of the magnetic and electric fields. The calculation of the Ar was done very well.
The question on the structure of atoms generally scored well. The workings of the mass spectrometer was less well known; with many getting confused with the role of the magnetic and electric fields. The calculation of the Ar was done very well.
State the relative mass and charge of the subatomic particles of an atom.

(i) Calculate the number of neutrons and electrons in one atom of \(^{{\text{65}}}{\text{Cu}}\).
Neutrons:
Electrons:
(ii) State one difference in the physical properties of the isotopes \(^{{\text{63}}}{\text{Cu}}\) and \(^{{\text{65}}}{\text{Cu}}\) and explain why their chemical properties are the same.
Physical:
Chemical:
Describe the bonding in solid copper.
Suggest two properties of copper that make it useful and economically important.
Markscheme
 ;
;
Award [2] for all four correct.
Award [1] for two or three correct.
(i) Neutrons: 36 and Electrons: 29;
(ii) Physical:
\(^{{\text{63}}}{\text{Cu}}\) lower boiling point/melting point/density/greater rate of diffusion than \(^{{\text{65}}}{\text{Cu}}\);
Accept converse arguments.
Do not accept “different mass”.
Chemical:
(properties identical because) same electron configuration/arrangement of electrons;
Accept “same number of protons and electrons”.
Do not accept “same number of electrons” OR “same valence (electrons)”
OR “same atomic number” only.
electrostatic attraction;
between (a lattice of) cations/positive ions and delocalized/sea of electrons;
Do not award any mark for only stating “metallic bonding”.
Award [1] for any two of:
malleable / ductile / conducts electricity / conducts heat / durable / strong / resistant to corrosion / low reactivity;
Examiners report
This question focused on atomic structure and metals was very accessible. Many students could correctly state relative mass and charge of the subatomic particles but it was disappointing to see the number of students who could not. Units were not penalized which meant that more students gained marks than would otherwise have been the case. Many students could calculate the protons and neutrons in the copper isotope but few could explain how the physical properties would vary (mass is not a property) and few could clearly explain why the chemical properties were identical. Explanations were often far too vague. Two properties of copper were asked for in and many could give one, again answers were often very vague, such as “it conducts” without specifying what it was conducting.
This question focused on atomic structure and metals was very accessible. Many students could correctly state relative mass and charge of the subatomic particles but it was disappointing to see the number of students who could not. Units were not penalized which meant that more students gained marks than would otherwise have been the case. Many students could calculate the protons and neutrons in the copper isotope but few could explain how the physical properties would vary (mass is not a property) and few could clearly explain why the chemical properties were identical. Explanations were often far too vague. Two properties of copper were asked for in and many could give one, again answers were often very vague, such as “it conducts” without specifying what it was conducting.
This question focused on atomic structure and metals was very accessible. Many students could correctly state relative mass and charge of the subatomic particles but it was disappointing to see the number of students who could not. Units were not penalized which meant that more students gained marks than would otherwise have been the case. Many students could calculate the protons and neutrons in the copper isotope but few could explain how the physical properties would vary (mass is not a property) and few could clearly explain why the chemical properties were identical. Explanations were often far too vague. Two properties of copper were asked for in and many could give one, again answers were often very vague, such as “it conducts” without specifying what it was conducting.
This question focused on atomic structure and metals was very accessible. Many students could correctly state relative mass and charge of the subatomic particles but it was disappointing to see the number of students who could not. Units were not penalized which meant that more students gained marks than would otherwise have been the case. Many students could calculate the protons and neutrons in the copper isotope but few could explain how the physical properties would vary (mass is not a property) and few could clearly explain why the chemical properties were identical. Explanations were often far too vague. Two properties of copper were asked for in and many could give one, again answers were often very vague, such as “it conducts” without specifying what it was conducting.
Explain why the relative atomic mass of argon is greater than the relative atomic mass of potassium, even though the atomic number of potassium is greater than the atomic number of argon.
Deduce the numbers of protons and electrons in the \({{\text{K}}^ + }\) ion.
Markscheme
argon has a greater proportion of heavier isotopes / OWTTE / argon has a greater number of neutrons;
19 protons and 18 electrons;
Examiners report
This question was the best answered on the paper and generally well answered question. In part (a) candidates sometimes incorrectly used the term relative atomic mass instead of relative isotopic mass when referring to the mass of an isotope.
Most candidates correctly deduced the number of protons and electrons in the \({{\text{K}}^ + }\) ion, however some candidates did not read the question carefully and deduced the number of subatomic particles in the K atom.
The graph of the first ionization energy plotted against atomic number for the first twenty elements shows periodicity.

Strontium exists as four naturally-occurring isotopes. Calculate the relative atomic mass of strontium to two decimal places from the following data.

Define the term first ionization energy and state what is meant by the term periodicity.
State the electron arrangement of argon and explain why the noble gases, helium, neon and argon show the highest first ionization energies for their respective periods.
A graph of atomic radius plotted against atomic number shows that the atomic radius decreases across a period. Explain why chlorine has a smaller atomic radius than sodium.
Explain why a sulfide ion, \({{\text{S}}^{2 - }}\), is larger than a chloride ion, \({\text{C}}{{\text{l}}^ - }\).
Explain why the melting points of the Group 1 metals \({\text{(Li}} \to {\text{Cs)}}\) decrease down the group whereas the melting points of the Group 7 elements \({\text{(F}} \to {\text{I)}}\) increase down the group.
Markscheme
\({A_{\text{r}}} = \frac{{[(0.56 \times 84) + (9.90 \times 86) + (7.00 \times 87) + (82.54 \times 88)]}}{{100}}\);
\( = 87.71\);
Award [1 max] if answer not given to two decimal places.
Award [2] for correct final answer.
Apply –1(U) if answer quoted in g or g\(\,\)mol–1.
first ionization energy: \({\text{M(g)}} \to {{\text{M}}^ + }{\text{(g)}} + {{\text{e}}^ - }{\text{/e}}\) / the (minimum) energy (\({\text{in kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)) to remove one electron from a gaseous atom / the energy required to remove one mole of electrons from one mole of gaseous atoms;
periodicity: repeating pattern of (physical and chemical) properties;
2.8.8/sp version;
Accept any two of the following:
the outer energy level/shell is full;
the increased charge on the nucleus;
great(est) attraction for electrons;
17 p in Cl nucleus attract the outer level more than 11 p in Na nucleus / greater nuclear charge attracts outer level more;
Allow converse for Na.
Do not accept larger nucleus.
\({{\text{S}}^{2 - }}\) has one proton less/ smaller nuclear charge so outer level held less strongly / OWTTE;
Allow converse for chloride.
Do not accept larger nucleus.
the radii of the metal atoms increase (from \({\text{Li}} \to {\text{Cs}}\)) (so the forces of attraction are less between them) / OWTTE;
the forces of attraction between halogen molecules are van der Waals;
forces increase with increasing mass/number of electrons;
Examiners report
The type of calculation in (a)(iii)is well understood. In the cases where both marks were not awarded, this was due to arithmetic error, not reading the question properly and providing an answer to other than two decimal places, or giving some unit such as grams.
Part (b)(i) resulted in very few marks being awarded; the requirements to refer to a gaseous atom and the idea of repetition in periodicity were sufficient to prevent otherwise reasonable answers from scoring.
The electron configuration was usually known in (b)(ii), as was the fact that there is a full outer electron shell. The third mark was less frequently awarded, with candidates often using the simplistic ‘it is full so it doesn’t want to lose electrons’ argument.
(b)(iii) was commonly correct but (b)(iv) was less well answered as many candidates failed to realise that these ions are isoelectronic and gave an answer relating to sulphide having more electrons with a consequent increase in repulsions.
(b)(iii) was commonly correct but (b)(iv) was less well answered as many candidates failed to realise that these ions are isoelectronic and gave an answer relating to sulphide having more electrons with a consequent increase in repulsions.
Part (b)(v) was poorly answered. Very few scored the first mark, many answers referring to some sort of ionization process. A handful of candidates scored the second mark for a reference to van der Waals forces but explanations for an increase were very weak. A number of candidates lost marks by referring to the breaking of covalent bonds rather than overcoming intermolecular forces.
Magnesium is a group 2 metal which exists as a number of isotopes and forms many compounds.
State the nuclear symbol notation, \({}_Z^AX\), for magnesium-26.
Mass spectroscopic analysis of a sample of magnesium gave the following results:
Calculate the relative atomic mass, Ar, of this sample of magnesium to two decimal places.
Magnesium burns in air to form a white compound, magnesium oxide. Formulate an equation for the reaction of magnesium oxide with water.
Describe the trend in acid-base properties of the oxides of period 3, sodium to chlorine.
In addition to magnesium oxide, magnesium forms another compound when burned in air. Suggest the formula of this compound
Describe the structure and bonding in solid magnesium oxide.
Magnesium chloride can be electrolysed.
Deduce the half-equations for the reactions at each electrode when molten magnesium chloride is electrolysed, showing the state symbols of the products. The melting points of magnesium and magnesium chloride are 922 K and 987 K respectively.
Anode (positive electrode):
Cathode (negative electrode):
Markscheme
\({}_{12}^{26}{\rm{Mg}}\)
«Ar =»\(\frac{{24 \times 78.60 + 25 \times 10.11 + 26 \times 11.29}}{{100}}\)
«= 24.3269 =» 24.33
Award [2] for correct final answer.
Do not accept data booklet value (24.31).
MgO(s) + H2O(l) → Mg(OH)2(s)
OR
MgO(s) + H2O(l) → Mg2+(aq) + 2OH–(aq)
Accept \( \rightleftharpoons \).
from basic to acidic
through amphoteric
Accept “alkali/alkaline” for “basic”.
Accept “oxides of Na and Mg: basic AND oxide of Al: amphoteric” for M1.
Accept “oxides of non-metals/Si to Cl acidic” for M2.
Do not accept just “become more acidic”
Mg3N2
Accept MgO2, Mg(OH)2, Mg(NOx)2, MgCO3.
«3-D/giant» regularly repeating arrangement «of ions»
OR
lattice «of ions»
Accept “giant” for M1, unless “giant covalent” stated.
electrostatic attraction between oppositely charged ions
OR
electrostatic attraction between Mg2+ and O2– ions
Do not accept “ionic” without description.
Anode (positive electrode):
2Cl– → Cl2(g) + 2e–
Cathode (negative electrode):
Mg2+ + 2e– → Mg(l)
Penalize missing/incorrect state symbols at Cl2 and Mg once only.
Award [1 max] if equations are at wrong electrodes.
Accept Mg (g).
Examiners report
There are many oxides of silver with the formula AgxOy. All of them decompose into their elements when heated strongly.
After heating 3.760 g of a silver oxide 3.275 g of silver remained. Determine the empirical formula of AgxOy.
Suggest why the final mass of solid obtained by heating 3.760 g of AgxOy may be greater than 3.275 g giving one design improvement for your proposed suggestion. Ignore any possible errors in the weighing procedure.
Naturally occurring silver is composed of two stable isotopes, 107Ag and 109Ag.
The relative atomic mass of silver is 107.87. Show that isotope 107Ag is more abundant.
Some oxides of period 3, such as Na2O and P4O10, react with water. A spatula measure of each oxide was added to a separate 100 cm3 flask containing distilled water and a few drops of bromothymol blue indicator.
The indicator is listed in section 22 of the data booklet.
Deduce the colour of the resulting solution and the chemical formula of the product formed after reaction with water for each oxide.
Explain the electrical conductivity of molten Na2O and P4O10.
Outline the model of electron configuration deduced from the hydrogen line emission spectrum (Bohr’s model).
Markscheme
n(Ag) = «\(\frac{{3.275{\text{ g}}}}{{107.87{\text{ g}}\,{\text{mol}}}} = \)» 0.03036 «mol»
AND
n(O) = «\(\frac{{3.760{\text{ g}} - 3.275{\text{ g}}}}{{16.00{\text{ g}}\,{\text{mo}}{{\text{l}}^{ - 1}}}} = \frac{{0.485}}{{16.00}} = \)» 0.03031 «mol»
«\(\frac{{0.03036}}{{0.03031}} \approx 1\) / ratio of Ag to O approximately 1 : 1, so»
AgO
Accept other valid methods for M1.
Award [1 max] for correct empirical formula if method not shown.
[2 marks]
temperature too low
OR
heating time too short
OR
oxide not decomposed completely
heat sample to constant mass «for three or more trials»
Accept “not heated strongly enough”.
If M1 as per markscheme, M2 can only be awarded for constant mass technique.
Accept "soot deposition" (M1) and any suitable way to reduce it (for M2).
Accept "absorbs moisture from atmosphere" (M1) and "cool in dessicator" (M2).
Award [1 max] for reference to impurity AND design improvement.
[2 marks]
Ar closer to 107/less than 108 «so more 107Ag»
OR
Ar less than the average of (107 + 109) «so more 107Ag»
Accept calculations that gives greater than 50% 107Ag.
[1 mark]
Do not accept name for the products.
Accept “Na+ + OH–” for NaOH.
Ignore coefficients in front of formula.
[3 marks]
«molten» Na2O has mobile ions/charged particles AND conducts electricity
«molten» P4O10 does not have mobile ions/charged particles AND does not conduct electricity/is poor conductor of electricity
Do not award marks without concept of mobile charges being present.
Award [1 max] if type of bonding or electrical conductivity correctly identified in each compound.
Do not accept answers based on electrons.
Award [1 max] if reference made to solution.
[2 marks]
electrons in discrete/specific/certain/different shells/energy levels
energy levels converge/get closer together at higher energies
OR
energy levels converge with distance from the nucleus
Accept appropriate diagram for M1, M2 or both.
Do not give marks for answers that refer to the lines in the spectrum.
[2 marks]
Examiners report
Titanium is a transition metal.
TiCl4 reacts with water and the resulting titanium(IV) oxide can be used as a smoke screen.
Describe the bonding in metals.
Titanium exists as several isotopes. The mass spectrum of a sample of titanium gave the following data:
Calculate the relative atomic mass of titanium to two decimal places.
State the number of protons, neutrons and electrons in the \({}_{22}^{48}{\text{Ti}}\) atom.
State the full electron configuration of the \({}_{22}^{48}{\text{Ti}}\)2+ ion.
Explain why an aluminium-titanium alloy is harder than pure aluminium.
State the type of bonding in potassium chloride which melts at 1043 K.
A chloride of titanium, TiCl4, melts at 248 K. Suggest why the melting point is so much lower than that of KCl.
Formulate an equation for this reaction.
Suggest one disadvantage of using this smoke in an enclosed space.
Markscheme
electrostatic attraction
between «a lattice of» metal/positive ions/cations AND «a sea of» delocalized electrons
Accept mobile electrons.
Do not accept “metal atoms/nuclei”.
[2 marks]
\(\frac{{(46 \times 7.98) + (47 \times 7.32) + (48 \times 73.99) + (49 \times 5.46) + (50 \times 5.25)}}{{100}}\)
= 47.93
Answer must have two decimal places with a value from 47.90 to 48.00.
Award [2] for correct final answer.
Award [0] for 47.87 (data booklet value).
[2 marks]
Protons: 22 AND Neutrons: 26 AND Electrons: 22
[1 mark]
1s22s22p63s23p63d2
[1 mark]
titanium atoms/ions distort the regular arrangement of atoms/ions
OR
titanium atoms/ions are a different size to aluminium «atoms/ions»
prevent layers sliding over each other
Accept diagram showing different sizes of atoms/ions.
[2 marks]
ionic
OR
«electrostatic» attraction between oppositely charged ions
[1 mark]
«simple» molecular structure
OR
weak«er» intermolecular bonds
OR
weak«er» bonds between molecules
Accept specific examples of weak bonds such as London/dispersion and van der Waals.
Do not accept “covalent”.
[1 mark]
TiCl4(l) + 2H2O(l) → TiO2(s) + 4HCl(aq)
correct products
correct balancing
Accept ionic equation.
Award M2 if products are HCl and a compound of Ti and O.
[2 marks]
HCl causes breathing/respiratory problems
OR
HCl is an irritant
OR
HCl is toxic
OR
HCl has acidic vapour
OR
HCl is corrosive
Accept “TiO2 causes breathing problems/is an irritant”.
Accept “harmful” for both HCl and TiO2.
Accept “smoke is asphyxiant”.
[1 mark]
Examiners report
The emission spectrum of an element can be used to identify it.
Elements show trends in their physical properties across the periodic table.
Draw the first four energy levels of a hydrogen atom on the axis, labelling n = 1, 2, 3 and 4.
Draw the lines, on your diagram, that represent the electron transitions to n = 2 in the emission spectrum.
Outline why atomic radius decreases across period 3, sodium to chlorine.
Outline why the ionic radius of K+ is smaller than that of Cl−.
Copper is widely used as an electrical conductor.
Draw arrows in the boxes to represent the electronic configuration of copper in the 4s and 3d orbitals.
Impure copper can be purified by electrolysis. In the electrolytic cell, impure copper is the anode (positive electrode), pure copper is the cathode (negative electrode) and the electrolyte is copper(II) sulfate solution.
Formulate the half-equation at each electrode.
Outline where and in which direction the electrons flow during electrolysis.
Markscheme

4 levels showing convergence at higher energy
[1 mark]
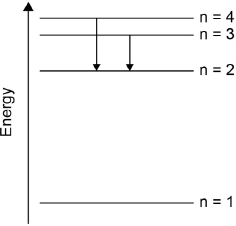
arrows (pointing down) from n = 3 to n = 2 AND n = 4 to n = 2
[1 mark]
same number of shells/«outer» energy level/shielding AND nuclear charge/number of protons/Zeff increases «causing a stronger pull on the outer electrons»
[1 mark]
K+ 19 protons AND Cl– 17 protons
OR
K+ has «two» more protons
same number of electrons/isoelectronic «thus pulled closer together»
[2 marks]

[1 mark]
Anode (positive electrode):
Cu(s) → Cu2+(aq) + 2e–
Cathode (negative electrode):
Cu2+(aq) + 2e– → Cu(s)
Accept Cu(s) – 2e– → Cu2+(aq).
Accept \( \rightleftharpoons \) for →
Award [1 max] if the equations are at the wrong electrodes.
[2 marks]
«external» circuit/wire AND from positive/anode to negative/cathode electrode
Accept “through power supply/battery” instead of “circuit”.
[1 mark]
Examiners report
Trends in physical and chemical properties are useful to chemists.
The Activity series lists the metal in order of reactivity.
Explain the general increasing trend in the first ionization energies of the period 3 elements, Na to Ar.
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group.
State an equation for the reaction of phosphorus (V) oxide, P4O10 (s), with water.
Describe the emission spectrum of hydrogen.
Identify the strongest reducing agent in the given list.
A voltaic cell is made up of a Mn2+/Mn half-cell and a Ni2+/Ni half-cell.
Deduce the equation for the cell reaction.
The voltaic cell stated in part (ii) is partially shown below.
Draw and label the connections needed to show the direction of electron movement and ion flow between the two half-cells.
Markscheme
increasing number of protons
OR
increasing nuclear charge
«atomic» radius/size decreases
OR
same number of shells
OR
similar shielding «by inner electrons»
«greater energy needed to overcome increased attraction between nucleus and electrons»
atomic/ionic radius increases
smaller charge density
OR
force of attraction between metal ions and delocalised electrons decreases
Do not accept discussion of attraction between valence electrons and
nucleus for M2.
Accept “weaker metallic bonds” for M2.
P4O10 (s) + 6H2O (l) → 4H3PO4 (aq)
Accept “P4O10 (s) + 2H2O (l) → 4HPO3 (aq)” (initial reaction).
«series of» lines
OR
only certain frequencies/wavelengths
convergence at high«er» frequency/energy/short«er» wavelength
M1 and/or M2 may be shown on a diagram.
Mn
Mn (s) + Ni2+ (aq) → Ni (s) + Mn2+ (aq)
wire between electrodes AND labelled salt bridge in contact with both electrolytes
anions to right (salt bridge)
OR
cations to left (salt bridge)
OR
arrow from Mn to Ni (on wire or next to it)
Electrodes can be connected directly or through voltmeter/ammeter/light bulb, but not a battery/power supply.
Accept ions or a specific salt as the label of the salt bridge.
Examiners report
Alkenes are widely used in the production of polymers. The compound A, shown below, is used in the manufacture of synthetic rubber.
(i) State the name, applying IUPAC rules, of compound A.
(ii) Draw a section, showing three repeating units, of the polymer that can be formed from compound A.
(iii) Compound A is flammable. Formulate the equation for its complete combustion.
Compound B is related to compound A.
(i) State the term that is used to describe molecules that are related to each other in the same way as compound A and compound B.
(ii) Suggest a chemical test to distinguish between compound A and compound B, giving the observation you would expect for each.
Test:
Observation with A:
Observation with B:
(iii) Spectroscopic methods could also be used to distinguish between compounds A and B.
Predict one difference in the IR spectra and one difference in the 1H NMR spectra of these compounds, using sections 26 and 27 of the data booklet.
IR spectra:
1H NMR spectra:
A sample of compound A was prepared in which the 12C in the CH2 group was replaced by 13C.
(i) State the main difference between the mass spectrum of this sample and that of normal compound A.
(ii) State the structure of the nucleus and the orbital diagram of 13C in its ground state.
Draw a 1s atomic orbital and a 2p atomic orbital.
Markscheme
(i)
methylpropene
(ii)
−CH2−C(CH3)2−CH2−C(CH3)2−CH2−C(CH3)2−
Must have continuation bonds at both ends.
Accept any orientation of the monomers, which could give methyl side-chains on neighbouring atoms etc.
(iii)
C4H8 (g) + 6O2 (g) → 4CO2 (g) + 4H2O(l)
(i)
«structural/functional group» isomer«s»
(ii)
Test:
«react with» bromine/Br2 «in the dark»
OR
«react with» bromine water/Br2 (aq) «in the dark»
IR: A would absorb at 1620–1680cm−1 AND B would not
1H NMR: A would have 2 signals AND B would have 1 signal
OR
A would have a signal at 4.5–6.0 ppm AND B would not
OR
A would have a signal at 0.9–1.0 ppm AND B would not
OR
B would have a signal at 1.3–1.4 ppm AND A would not
Accept “peak” for “signal”.
Award [1 max] if students have a correct assignation of a signal, but no comparison, for both IR and NMR.
Accept “B would have a signal at 2.0 ppm” as shown in its 1H NMR spectrum.
(i)
«molecular ion» peak at «m/z =» 57, «not 56»
OR
«molecular ion» peak at one «m/z» higher
OR
will not have a «large» peak at 56
Accept a peak at m/z one greater than the 12C one for any likely fragment.
(ii)
protons: 6 AND neutrons: 7
Accept full arrows.
Accept p orbitals aligned on y− and z−axes, or diagrams correctly showing all three p-orbitals.
Do not accept p-orbitals without a node.